Oral Suspension, USP
Diazoxide Oral Suspension, USP
Diazoxide is a nondiuretic benzothiadiazine derivative taken orally for the management of symptomatic hypoglycemia. Diazoxide Oral Suspension, USP contains 50 mg of diazoxide, USP in each milliliter and has a chocolate-mint flavor; alcohol content is approximately 7.25%.
Other ingredients: Sorbitol solution, chocolate mint flavor, propylene glycol, magnesium aluminum silicate, carboxymethycellulose sodium, sodium benzoate, methylparaben, poloxamer 188, propylparaben, and purified water. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
Diazoxide is 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide with the empirical formula C8H7ClN2O2S and the molecular weight 230.7. It is a white powder practically insoluble to sparingly soluble in water.
Indications and Usage
Diazoxide Oral Suspension, USP is useful in the management of hypoglycemia due to hyperinsulinism associated with the following conditions:
Infants and Children: Leucine sensitivity, islet cell hyperplasia, nesidioblastosis, extrapancreatic malignancy, islet cell adenoma, or adenomatosis. Diazoxide Oral Suspension, USP may be used preoperatively as a temporary measure, and postoperatively, if hypoglycemia persists.
Diazoxide should be used only after a diagnosis of hypoglycemia due to one of the above conditions has been definitely established. When other specific medical therapy or surgical management either has been unsuccessful or is not feasible, treatment with diazoxide should be considered.


Patient Information
During treatment with diazoxide the patient should be advised to consult regularly with the physician and to cooperate in the periodic monitoring of his or her condition by laboratory tests. In addition, the patient should be advised:
- to take the drug on a regular schedule as prescribed, not to skip doses, not to take extra doses;
- not to use this drug with other medications unless this is done with the physician’s advice;
- not to allow anyone else to take this medication;
- to follow dietary instructions;
- to report promptly any adverse effects (i.e., increased urinary frequency, increased thirst, fruity breath odor);
- to report pregnancy or to discuss plans for pregnancy.
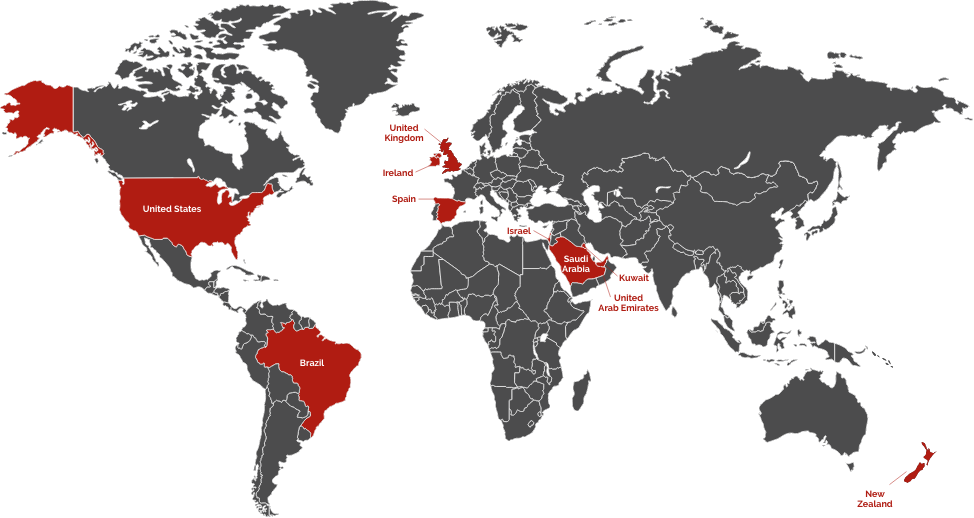
Regions Where e5 Pharma Diazoxide Oral Suspension is Sold: